Know thy enemy
Let's start with an overview
We suggest you first get an overview by having a look at this video:
There are two main outputs from this video, what we have called the KTE Model, and the KTE roadmap.
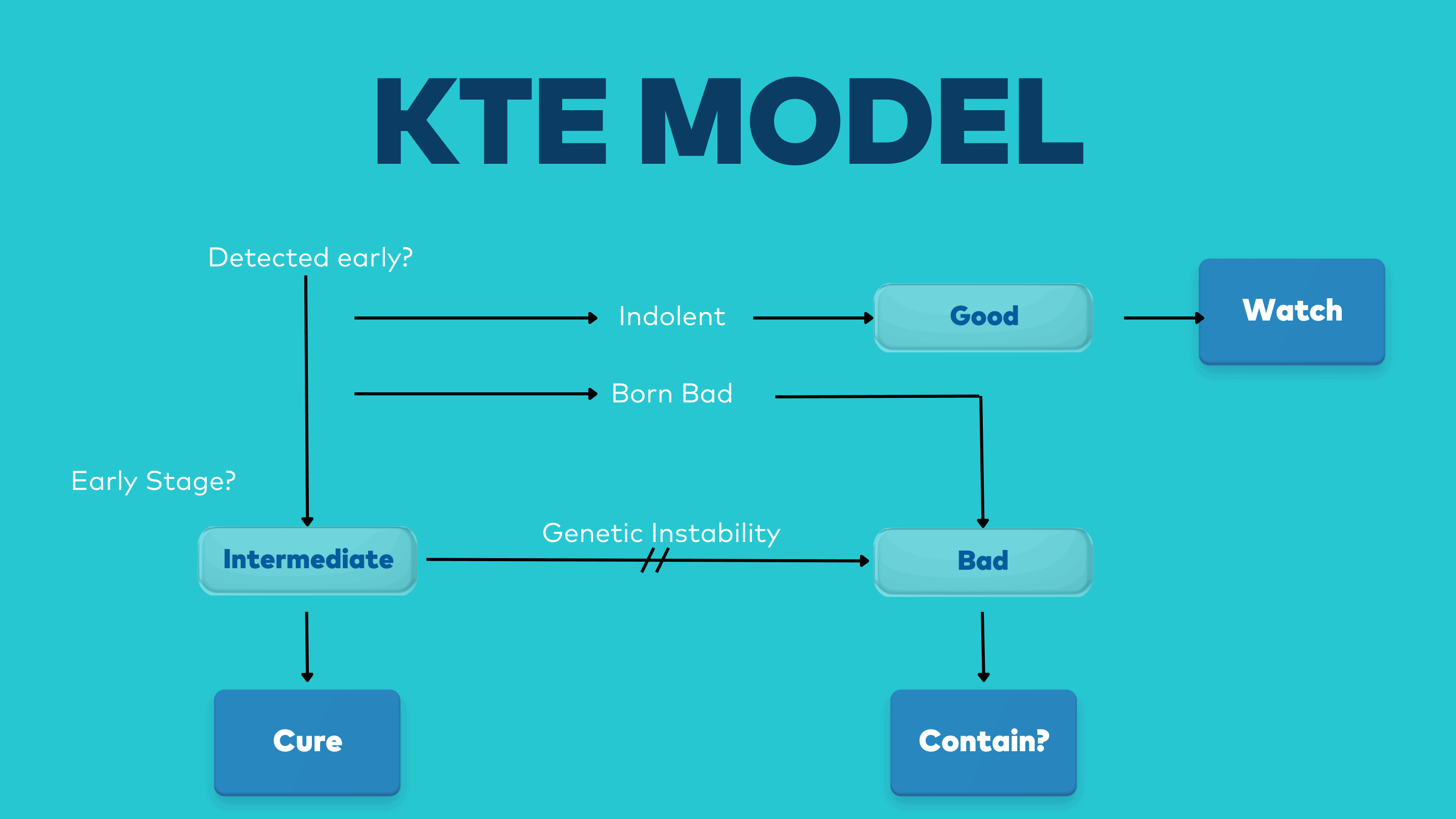
The KTE model shows, in broad terms, how each of the three classes of cancer arise, each with their main treatment objective.
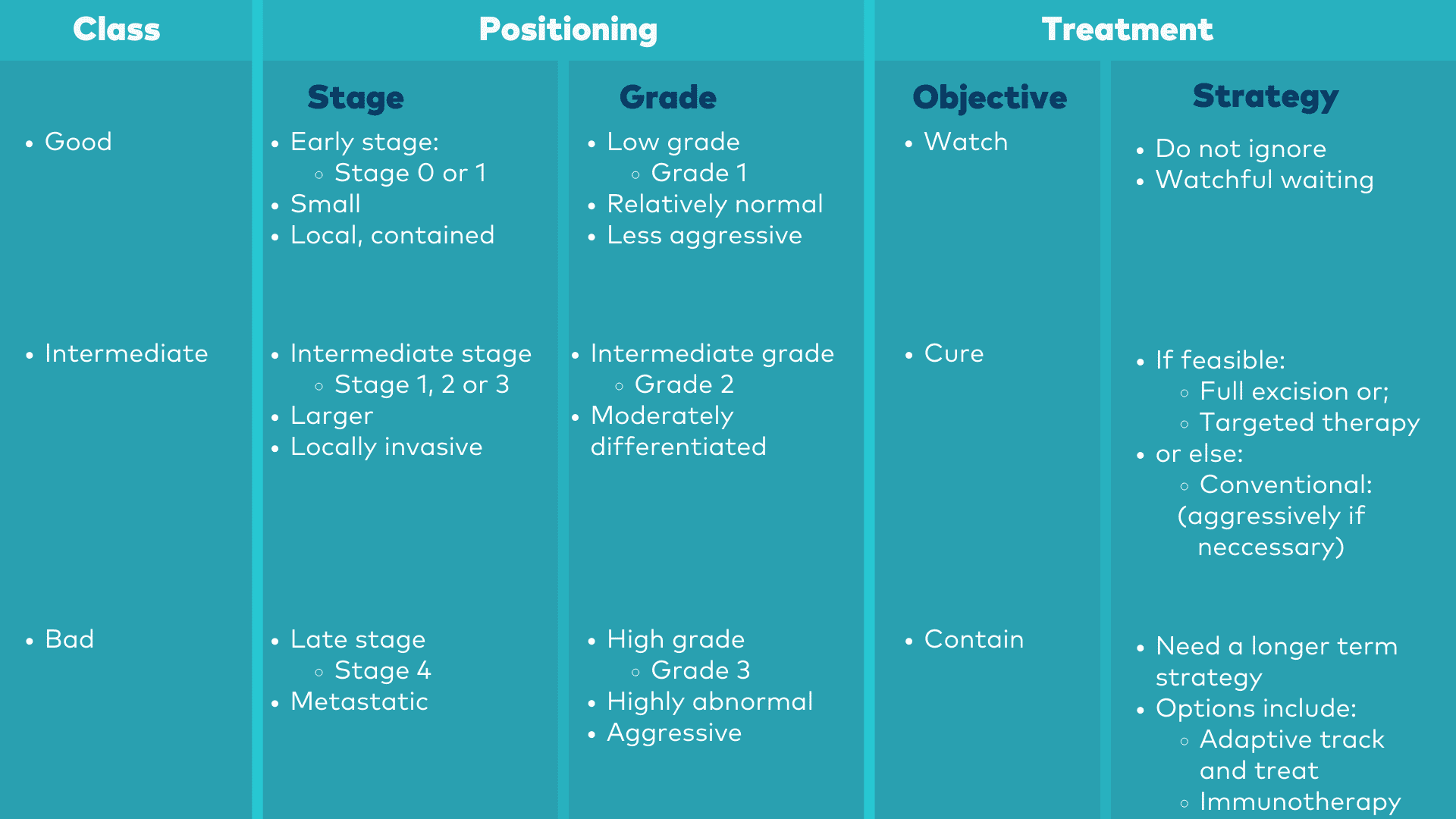
The KTE roadmap helps you to position a particular cancer in relation to the three classes, along with treatment implications.
The KTE Model
The KTE Roadmap
So how then do you proceed for your own particular cancer?
The first step is to determine in which class your cancer belongs; that is, you need to "Position" your cancer. As we will describe later below, that primarily requires two key numbers which your doctor will get from your pathology report, which are the stage and the grade of your cancer. With these two numbers you can then identify the class of your cancer from the roadmap above,
Once it is positioned, you can then focus in on your own particular class of cancer:
A GOOD cancer is usually found during early detection, or as an
accidental result of some other procedure such as an examination or a scan.
It is potentially indolent; very slow growing, and unlikely to
progress to a high-grade malignancy during an average human life span.
In terms of positioning it is early stage and low grade.
It should not be treated and should rather be actively WATCHED.
INTERMEDIATES are earlier stage cancers which are not indolent and
need to be treated.
It has moderately abnormal cells (medium grade); is relatively stable
(mutating and evolving slowly); and homogeneous.
It is therefore unlikely to develop any significant resistance to
treatment.
Intermediates are eminently treatable and should be treated with the
objective of CURE.
BAD cancers usually arise from intermediates that are either not
treated or else recur following treatment
(though there are some bad cancers that are genetically unstable from birth, so
called Born to be Bads).
Bad cancers are high grade; genetically unstable; heterogeneous; and
evolving fast.
They are late stage and more than likely metastatic.
Bad cancers are therefore highly resistant to treatment and very
difficult to treat.
They have limited chance of cure and should be treated with the
objective to CONTAIN.
(Note, however, we will show that the possibility of some cures is starting to emerge in some cases).
So now .....
If you are in a hurry to learn more about your particular class of cancer, then just click on the appropriate link (in blue above) for your class.
If you would prefer to rather understand more about cancer first, then you can continue reading.
What exactly is Cancer?
Cancer is a disease which starts when damaged cells divide without control. Cancer is essentially a disease of
the genes. In broad terms, this is how it happens:
The cells of our body are constantly at work. They need to
grow and divide in order to maintain and repair the body. In normal
circumstances this occurs under the strict control of processes within the
cells and their surrounding environment.
Cancer occurs when some of the genes driving and controlling
these processes get damaged (mutated) and start dividing uncontrollably.
There are two types of genes which both need to be damaged
for cancer to be initiated; drivers of cell division, the so-called proto-oncogenes
(foot stuck on the accelerator); and controllers against excessive
proliferation, the tumour suppressor genes (brakes fail). There are two
important consequences of this:
1. More than one mutation in cancer-inducing genes
is required to initiate cancerous growth, and
2. Every cancer starts as an expansion from a
single damaged (rebel) cell.
How Cancer cells behave
But there are other important features which cancers develop
as they progress:
Firstly, there is malignancy, and it is a major reason that
some cancers are so very dangerous. A malignant cancer is one which has invaded
nearby tissue and possibly spread (metastasised) to other parts of the body to
form new tumours.
Furthermore, as cancers advance, they become heterogeneous. At the outset all cancers are genetically homogeneous, all cancer cells sharing the same mutations. See this as the trunk of a tree. But eventually, at some point a Rubicon is reached (in some tumours a lot sooner than others) when one of the tumour cells becomes genetically unstable. The clone growing from that unstable cell then starts mutating at a much faster rate and the tree starts branching. The cancer is then heterogeneous and evolving, and far more difficult to treat.
It is also important to understand that the immune system is
not blind to cancer. It can exert control over the growth of tumours. Indeed, in some cases it can eliminate
tumours. However, as cancers progress, they often evolve defences as protection
against the immune system.
Cancers form a complex and highly variable group of
diseases:
The number of genes that, when mutated, can contribute to cancer, run into the hundreds (and is growing); and each gene can be mutated in a number of ways. In any given patient, a subset of these genes must be mutated for the cancer to progress, a process that occurs sequentially over a number of years. The number of possible combinations of such mutations is thus huge. In effect, cancer is not one disease - but a cluster of diseases with hundreds of subtypes.
Indeed, at the genetic level, each and every cancer is
unique.
Traditional types of Cancer
Firstly, there are two main categories of cancer: hematologic
(blood) cancers such as leukaemia; and solid cancers such as breast and
prostate cancers. Surgery, for instance, is not an option for blood cancers.
The next level of classification is that of the main groups
of cancer. For example, a common split is between carcinoma (external and
internal surface lining), sarcoma (supportive and connective tissue including muscle
and bone), leukaemia (blood), and lymphoma (lymphatic system). See, for
example: https://www.cancer.gov/about-cancer/understanding/what-is-cancer
and click on types of cancer,
If you want to gain an understanding of your specific cancer
type, we recommend the National Cancer Institutes A-Z listing contained in https://www.cancer.gov/types, where
over 100 specific cancers are described.
In terms of treatment, there are 3 main classes of cancer
Cancer is not cancer is not cancer. It’s like there is
cancer and then there is CANCER. That is why it is important for you to
understand your particular cancer. You need to understand exactly what you are
dealing with.
Good, Intermediate and Bad
Start by recognising that, from a treatment point
of view, there are three main classes of cancer. This is not just our opinion.
Here is a quote from the leading textbook on cancer (Robert A. Weinberg, The
Biology of Cancer):
“The complexity of the decision to proceed with treatment
should ideally confront the fact that diagnosed tumours fall into three classes.”
In the book we draw off Weinberg’s work and, for
convenience, call these three classes the “Good”, the “Intermediates” and the
“Bad”.
Good Cancers
A “Good” cancer is usually found during early detection, or
as an accidental result of some other procedure such as an examination or a
scan. A “Good” cancer is one that is potentially indolent;
very slow growing, unlikely to progress to a high-grade malignancy during an
average human life span and need not be treated.
Intermediate Cancers
An “Intermediate” is one with moderately abnormal cells, is
genetically relatively stable (mutating and evolving slowly), homogeneous, and unlikely
to develop any significant resistance to treatment. An intermediate offers a good chance of cure.
Bad Cancers
A “Bad” cancer is advanced, genetically unstable (mutating
and evolving fast), heterogeneous and prone to resistance when treated. It is
probably also metastatic and has limited chance of cure.
So, although each cancer is unique, from a treatment point
of view there are also commonalities. What we now want to do is to identify the
key information you need to know in order to characterise your cancer from a
treatment point of view. We call this “positioning”.
Once you have this understanding you will be in a far better
position to discuss treatment options for your particular cancer with your
doctor.
Staging, Grading & Class
The first step is to determine which of the three treatment classes your cancer belongs to.
There are two numbers from your pathology report which are key to this classification: the stage of your cancer and the grade.
|
Class |
Stage |
Grade |
|
“Good” |
0 or 1 |
|
|
“Intermediate” |
1 to 3 |
2 |
|
“Bad” |
4 |
3 |
Your doctor will discuss your pathology report with you as
part of your diagnosis. This is a very important report and it is important that
you try to understand the relevance of what is in it. In particular, your
doctor will put a lot of emphasis on the stage and the grade of your cancer.
The specifics vary depending on the type of cancer, but in
broad terms:
Stage refers to the extent of your cancer, such as how large
the tumour is, and if and how far it has spread. The scale ranges from Stage 0
(a precancer), through stages 1 to 3 (cancer is present - the higher the
number, the larger the cancer tumour and the more it has invaded into nearby
tissues) to the highest, the notorious stage 4 (cancer has spread to distant
parts of the body, i.e., it is metastatic).
The grade of a cancer is based on the appearance of the
cancer cells under the microscope. It is a description of the tumour based on
how abnormal the tumour cells and the tumour tissue look under a microscope,
and is an indicator of how quickly a tumour is likely to grow and spread (aggression). Lower
grade cancer cells (grade 1) look relatively normal (well differentiated) and
are growing more slowly, grade 2 is intermediate grade (moderately
differentiated), while grade 3 is high grade, poorly differentiated and more
aggressive.
You can then map the stage and grade of your cancer to one
of the three classes we defined above. This is a critical step and warrants
discussion with your doctor. Note, however, that the terms “class of cancer”,
as well as “Good”, “Intermediate” and “Bad” cancers, are our own terms and not
terms a doctor will be familiar with. Nevertheless, they will help you shape your
discussion with your doctor.
This is an important milestone in the positioning of your cancer. But there are two other key indicators you should be looking for, especially if your cancer is “Intermediate” or “Bad”:
Is your cancer targetable?
Is your cancer immunogenic?
(Note
that if your class of cancer is “Good” then you can skip the rest of this section
and proceed immediately to your treatment options below)
Genetics: Is your Cancer targetable?
We saw that cancer is caused by mutation to genes controlling
the process of cell division. These are called driver genes. For some (by no
means all) cancers, driver genes can be identified for which specific targeted
drugs are available. These mutations are identified using a technology called
genetic sequencing and this is fast becoming a major addition to the armoury of
cancer diagnosis.
Targeted drugs are highly specific to the driver mutations and
therefore have fewer side effects. Another advantage is that they can often be
taken in pill form. This is a fairly recent addition to the cancer armoury with
the first targeted drug approved in 2001.
Targeted therapies are particularly useful if your cancer is
an “Intermediate” (which is homogeneous, and stable and therefore less likely
to develop resistance), but can also be useful in some “Bad” cancers.
The need and the possibility for the genetic sequencing of
your tumour is a conversation you definitely should have with your doctor. It
is important that you raise this – in the book we report recent surveys which
show that less than 50% of community-based oncologists are using genetic
testing to guide patient discussions about treatment in the US (in contrast
with over 70% of academic clinicians). You may do a bit of research before-hand – Google
“targeted therapy" for your cancer type.
If your cancer is sequenced then, not only can you look at
possible targets, but you can also get a feel for how mutated your cancer is (called
tumour mutational burden or TMB). High TMB can be an indicator of whether your
cancer is heterogeneous, but, more importantly, it is a measure of
immunogenicity, which we turn to next.
Is your Cancer Immunogenic?
Immunotherapy is the most recent addition to the cancer
treatment armoury. It doesn’t kill the cancer directly but rather releases the
brakes on the immune system so that the immune system can then kill the cancer. It
is an important addition because the immune system has
the capacity (in the book we use the concept of “variety”) to counter a
heterogeneous, evolving and metastatic cancer. And, indeed, it has already shown curative potential for a number of advanced, metastatic cancers.
So, it is also important for you to explore this option with your doctor, especially if you have a “bad” cancer. Ask your doctor whether there are any indications that the tumour may be visible to the immune system (immunogenic) and whether any further testing in this regard would be warranted.
Key indicators of immunogenicity are:
Tumour mutational burden
The tumour has a higher tumour mutational burden (TMB), and
hence a higher number of targets for the immune system to see. There are, for
example, cancers which have high TMB due to environmental damage (smoking or UV
damage to skin). But another important contributor to high TMB is damage to DNA
repair genes, either inherited or acquired; e.g., damaged BRCA genes, as well
as a condition called micro-satellite instability MSI, both of which can be
determined from genetic testing.
Immune checkpoint expression
A second indicator of immunogenicity is higher levels of
so-called immune checkpoint expression (especially the so-called PD-1 and PD-L1
checkpoint biomarkers); a measure of the level of the defences the tumour has
built up against immune system.
Tumour infiltrating lymphocytes
Another measure is that of so-called tumour infiltrating
lymphocytes (TIL’s). This looks at the presence and activity of the immune
cells in and around the tumour, and is used to determine if the tumour is hot
or cold (a hot tumour is more inflamed and has a higher presence and activity
of immune cells infiltrating the tumour, and this is a good indicator for the
use of immune checkpoint immunotherapy).
Immunotherapy is a very important and fast-growing new
treatment modality and is quite extensively covered in the book.
Once
all the information is available and your tumour is clearly positioned, you can
start looking at your treatment options with your doctor. We now look at the
more general, high-level treatment issues you could pursue with your doctor. There
is a separate section for each class of cancer and you need only read the
section appropriate to your specific class.
"Good" Cancer
A “Good” cancer is usually found during an early detection
process, or as an accidental result of some other procedure such as an
examination or a scan. It is potentially indolent.
Why do we say potentially? A (true) indolent is very slow
growing, unlikely to progress to a high-grade malignancy during an average
human life span and need not be treated. There is an issue, however. Currently,
early detection processes are not sufficiently precise to be able to
confidently discriminate between the “pussy cats” (true indolents) and the
“tigers” (the low grade, but potentially lethal growths that will, sooner or
later, progress).
There are two possible responses to this uncertainty:
“Just cut it out anyway, out of sight is out of mind”. However, this response comes with a significant risk of overtreatment, subjecting one to the unnecessary stress, trauma and cost of treating something that may never cause a significant problem (and, indeed, as we note in chapter 4 in the book, may even increase the risk of the cancer growing).
At the same time, it can’t just be ignored. Given the
uncertainty in identifying true indolents, there remains a risk that it will
progress. So, the alternative response is not to just ignore the tumour, but rather
to undertake a regular and on-going process of “watchful waiting”, in case it
starts turning nasty. Watchful waiting is a systematic and routine programme of
checking and testing to determine whether there is any evidence that the growth
is starting to progress (an example for prostate cancer can be found in the
book, chapter 4).
What to discuss with your doctor
So, if your cancer is “Good” (early-stage, low grade and potentially indolent) then here are some key issues for discussion with your doctor:
* What evidence is there that your tumour may be truly
indolent? Are there any concerns that it may not be? What are the probabilities?
* What would treatment to eliminate the tumour entail? What
are the benefits? What are the risks? Are there potential side effects, both
short term and long term?
* What would be involved in a watch-and-wait strategy? How would it be done and what would be the logistics?
* Would a ‘watch and wait’ strategy increase the risk or reduce the options if the tumour were to start progressing and had to be removed later?
With a treatment plan of ‘watch and wait’, there are also some
particular logistical issues:
* Make sure your health insurance would be on-side, preferably
through a chronic care arrangement.
* Discipline, discipline, discipline. Stick with the plan, meet your commitments, don’t ever feel that you are done and can discontinue the programme.
"Intermediate" Cancer
An “Intermediate” is a tumour with moderately abnormal cells.
It is genetically stable (mutating and evolving slowly), homogeneous, and unlikely
to develop any significant resistance to treatment. These cancers are eminently
treatable and offer a good chance of cure.
What does cure mean for an “intermediate”? If the cancer can
be fully excised through surgery, then game over.
If genetics identify a targetable driver mutation, the targeted therapy may well be a daily pill leading to long-term chronic containment of the disease, if not total elimination (with possible migration to a related but different pill if late resistance does occur).

If treated conventionally (some or all of surgery, radiation and chemotherapy), then treatment should, if necessary, err on the side of aggression lest some cancerous cells escape and start evolving into a more advanced cancer. Remember, the objective here should be total remission. (A case study can be found in the book illustrating such appropriate aggression – ‘Liza: Appropriate aggression in search of a cure’ in Chapter 5).
What to discuss with your doctor
Here, then, are some key questions you should be asking your
doctor if your cancer is in the intermediate class:
Get a clear understanding of the treatment objective. If the
objective is anything less than cure then challenge this and ask for reasons
why not. Get a second opinion if you feel unconvinced.
Is it possible to cut the tumour out? If so, is there any
risk that it can’t all be taken out? Should the surgery be supplemented with
any other treatment as insurance? Complete excision with surgery is first
prize, but remember, if just one cancer cell is left behind then the cancer
will likely recur (in the same way that the original cancer started for a
single cell).
If full excision is not possible, then ask about the
genetics? If genetic testing has not yet been done, then question this? If
testing has been done, then do your genetics show any of the known, targetable
mutations? What prognosis is foreseen with targeted treatment (noting that an
intermediate cancer is homogeneous and unlikely to develop significant
resistance to targeted treatment).
If it is not targetable, then the treatment proposal will
probably be conventional. Ask whether the proposed treatment is a standard
of care and the expected prognosis. And, as said before, if you feel there
is any uncertainty, then err on the side of aggression.
Immunotherapy is not yet standard for earlier stage cancers,
although trials are in progress. It is usually not necessary to consider a
trial for an “Intermediate” cancer.
The logistics of treating Intermediate Cancer
For an “Intermediate”, treatment should be relatively
standard and you can select an appropriate and convenient specialist from
private practice to lead your treatment; preferably one open to shared decision
making.
Under these circumstances, health insurance should not
present any issues, but try to keep them in the loop throughout.
"Bad" Cancer
A “Bad” cancer is advanced, genetically unstable (mutating
and evolving fast), heterogeneous and is prone to resistance when treated. It
is also likely to be metastatic. Cure with conventional treatments is not very likely.
“Bad” cancers need a different mind-set. They evolve
and change over time in response to treatment. You need to prepare for the long
haul. It’s a war rather than a battle. In your discussions with your doctor,
you should be thinking strategy, not just treatment.
What do we mean by strategy? In chapter 6 of the book, we
sketch out a strategy we call ‘active waiting’ as an example. This consists of
a process of adaptively controlling the cancer (rather than, probably futilely,
trying to aggressively destroy it), while keeping an active watch for a ‘next
new thing’ which may have curative potential for your particular cancer.[1]
[1] Remembering,
for instance, that the powerful new treatment of immunotherapy was a ‘next new
thing’ just 10 years ago, and the next phase of this modality, called
combination immunotherapy, is already well into clinical trials (see Chapter 9
in the book).

What to discuss with your doctor
The initial offering from your doctor will in all probability be the standard
of care for your cancer. Interrogate this approach, understand it, but don’t
just blindly accept it. What is the treatment objective? Is cure possible? What
is the 5-year survival rate? What are the main side-effects, both short term
and long term? What happens if (when) it stops working due to resistance?
Then explore other possible opportunities with your doctor:
Does your genetic testing show any of the more well-known
driver mutations? What role could targeted therapies play in your strategy?
Furthermore, targeted treatments could be used as part of
the control process of the ‘active waiting’ strategy on an adaptive ‘track and
treat’ basis (section 9.2 in the book for more on ‘track and treat’).
What is the medical evidence for immunotherapy for your type
of cancer? How immunogenic is your tumour? Is immunotherapy a serious option? (Remember
that immunotherapy has shown the potential to cure some advanced (“Bad”)
cancers; much more on this in chapters 6 and 9 in the book).
Once all this information is in place, then we propose one
more very important step before you make a final decision. Seriously consider
the option of a second opinion through an academic or research hospital. A
number of the larger such institutions in the US, for instance, offer this
service on a virtual basis (try googling “virtual second opinions for cancer”).
This will help broaden your options based on the latest
evidence for your specific cancer. As part of this process, you could also explore
the options arising from clinical trials. You could also explore the
possibility of an on-going virtual collaboration between your doctor and the academic/research
institution consulted, especially if your cancer is of research or academic
interest to them. If not of interest to them, ask whether they can identify
another institution where there could be such interest.
The logistics of treating a Bad Cancer
The logistics of your treatment also require a quantum shift
if your cancer is “Bad”.
We have already spoken of a possible collaboration with an
academic/research hospital. More than likely multiple specialities will be
involved in your treatment. In which case you could also consider being treated
in a one-stop shop for cancer care which could probably offer a better, more
co-ordinated treatment option.
You would also need to work closely with your health
insurance consultant (and a financial navigator at the hospital) to find out
what options they would or would not support. The treatment may have to be
adaptive, tracking the cancer as it evolves, requiring on-going participation
of the key parties, so try to keep an advisory team together.
Planning for the long haul
You may well also need to set up personal plans for the
long-haul and it helps to think logically about these:
- Possible relocation?
- A sound financial plan.
- Resources for emotional support.
- A support network.
If your cancer is “Bad”, don’t just jump into the first
option that is put on the table. You need to explore all options (and the
second opinion proposal will certainly help broaden the horizons). A war requires strategy and is not all about
just winning the next battle.
In researching the latest developments in immunotherapy,
Colin came to the conclusion that if he were to be diagnosed with an advanced
cancer then he would pursue immunotherapy (including possible trials arising from
the new horizon combination IO if necessary) as his preferred treatment option.
This argument is detailed in a blog: